What Is Usp Dissolution Test
The process is public health focused leveraging current science and technology and draws on the expertise of scientists and healthcare practitioners while providing. Pioneering and automating high-quality pharmaceutical testing solutions for dissolution disintegration hardness and other physical tests of tablets.

Dissolution Test Conditions For Usp Apparatus 2 And 3 Download Table
USP 32 General Notices3 General Notices and Requirements Change to read.

What is usp dissolution test. Developing USP General Chapter USP is a not-for-profit science-driven organization that has an established process for convening independent experts for the development and maintenance of quality standards. USP announces the approval of General Notices section 56030 Elemental Impurities in USP Drug Products and Dietary Supplements with an official date of December 1 2015This General Notices section will make applicable General Chapters Elemental ImpuritiesLimits and Elemental Contaminants in Dietary Supplements as of that date which reflects a delayed official date. Automation experts for tablet capsule dissolution testing USP 1256 flow-through dissolution USP 4 disintegration testing tablet hardness testers and automated sample preparation.
Dium when a monograph for the article is published in the compen- dium and an official date is generally or specifically assigned to the The General Notices and Requirements section the General monograph. Notices presents the basic assumptions definitions and defaultThe title specified in a monograph is the official.

Pdf Hydrodynamic Effects Of A Cannula In A Usp Dissolution Testing Apparatus 2 Semantic Scholar

Dissolution Test Apparatus And Types As Per Ip And Usp Very Important Topic Youtube

Tablet Dissolution Test In Different Stages S1 S2 And S3 Pharmaceutical Guidelines

The Influence Of Different Mechanical Stress On The Release Properties Of Hpmc Matrix Tablets In Sucrose Nacl Media Sciencedirect

Dissolution Apparatus And Its Type

Hydrodynamic Mass Transfer And Dissolution Effects Induced By Tablet Location During Dissolution Testing Journal Of Pharmaceutical Sciences
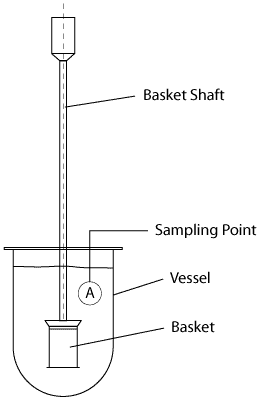
Apparatus 1 The Rotating Basket

Introduction To Dissolution Testing Accessories Methods
Usp31nf26s1 C711 General Chapters 711 Dissolution

Pdf Statistical Properties Of The Dissolution Test Of Usp Semantic Scholar

Dissolution Testing How Does It Work Youtube

Dissolution Testing For Osd Detect Physical Changes In Api And The Formulated Product Lls Health Cdmo

Dissolution Test Conditions Used For The Test Methods Download Table

Flow Through Cell Apparatus 4 Dissolution Tester Usp4 Sotax

Modi Fi Ed Usp Xxiii In Vitro Dissolution Testing Apparatus Download Scientific Diagram
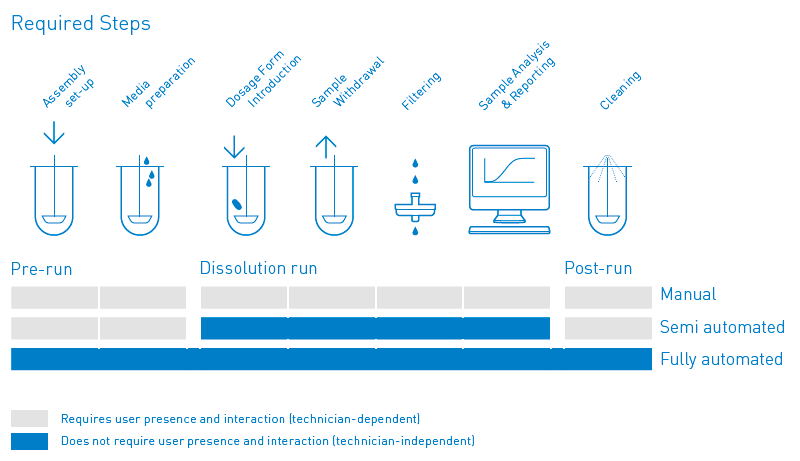
Dissolution Testing Usp 1 2 5 6

Dissolution Test And Apparatus Pharmaceutical Guidelines
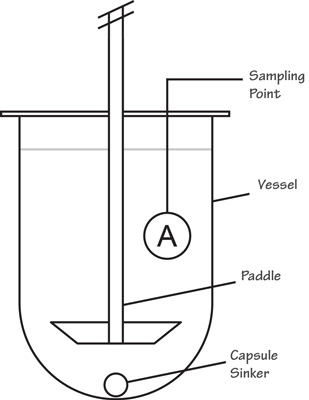
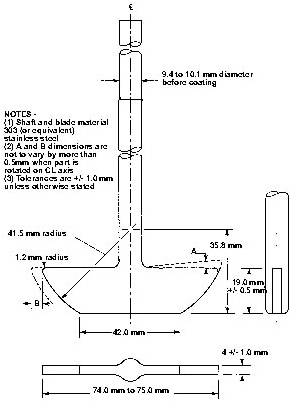
Posting Komentar untuk "What Is Usp Dissolution Test"